In our fifth article, Dr Cristina Florescu Moraid presents the history of GCP - the code for clinical trials, which are the pharma sponsors’ responsibilities according to these guidelines and the latest guidance to conduct clinical trials during the COVID 19 pandemic issued by EMA and FDA, the regulatory authorities in the US and Europe.
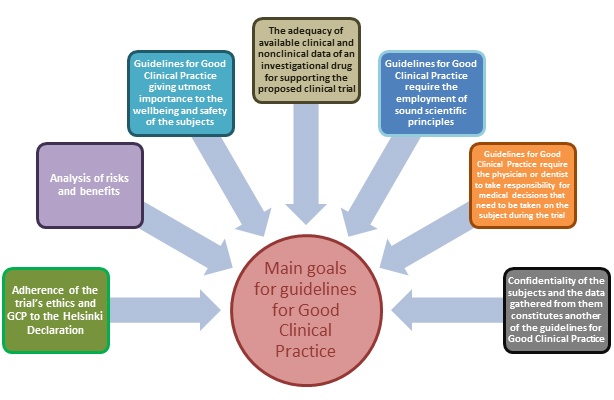
After the medical world has been shaken by tragic events with serious consequences for patients, with significant loss of life, it was concluded that any clinical research on humans should be closely observed and controlled, being carried out according to the GCP - Good Clinical code practice.
As a consequence of a joint initiative between the FDA (US Food and Drug Administration), the EMA (European Medicines Agency) and the Ministry of Health of Japan, the unique guideline for good clinical research - ICH-GCP (International Conference of Harmonization - Good Clinical Practice) was published in 1996.
The GCP guide explains the responsibilities of sponsors, monitors, investigators, ethics committees or competent authorities in each country where the clinical trials are conducted.
According to ICH – GCP, the sponsor has full responsibility for initiating, managing and financing the clinical trial. See detailed responsibilities in our article. More detailed responsibilities on the article.
On March 2020, the FDA and EMA have issued guidelines for conducting clinical trials during the COVID 19 pandemic, with recommendations on how to continue the ongoing clinical trials and to ensure that patient safety remains the main focus of clinical research during the pandemic.
The article was published on 3rd of April 2020, in Viata Medicala magazine no. 13, on page 6. SEE THE ARTICLE HERE .

***
The series of articles about clinical trials in Viata Medicala magazine in the section “Pastila de … studii clinice” / “The clinical trials … pill,” are part of our education and information activity in 2020. The articles are dedicated to physicians, especially those who have not yet come in contact with clinical trials.
Please check the News Section of our site to find the rest of the articles.
- We started a series of articles on clinical trials fundamentals & information in Viata Medicala magazine
- “The clinical trial, from A to Z”, an article in Viata Medicala magazine at the beginning of 2020
- About the actors of a clinical trial - Avantyo article in Viata Medicala Magazine
- “Patient-Centric Approach, the effective partnership” - Avantyo article inViata Medicala magazine
You might also find them posted on our social media pages, on Facebook and Linkedin.
Get news about our training programs directly in your inbox by subscribing to our newsletter!




