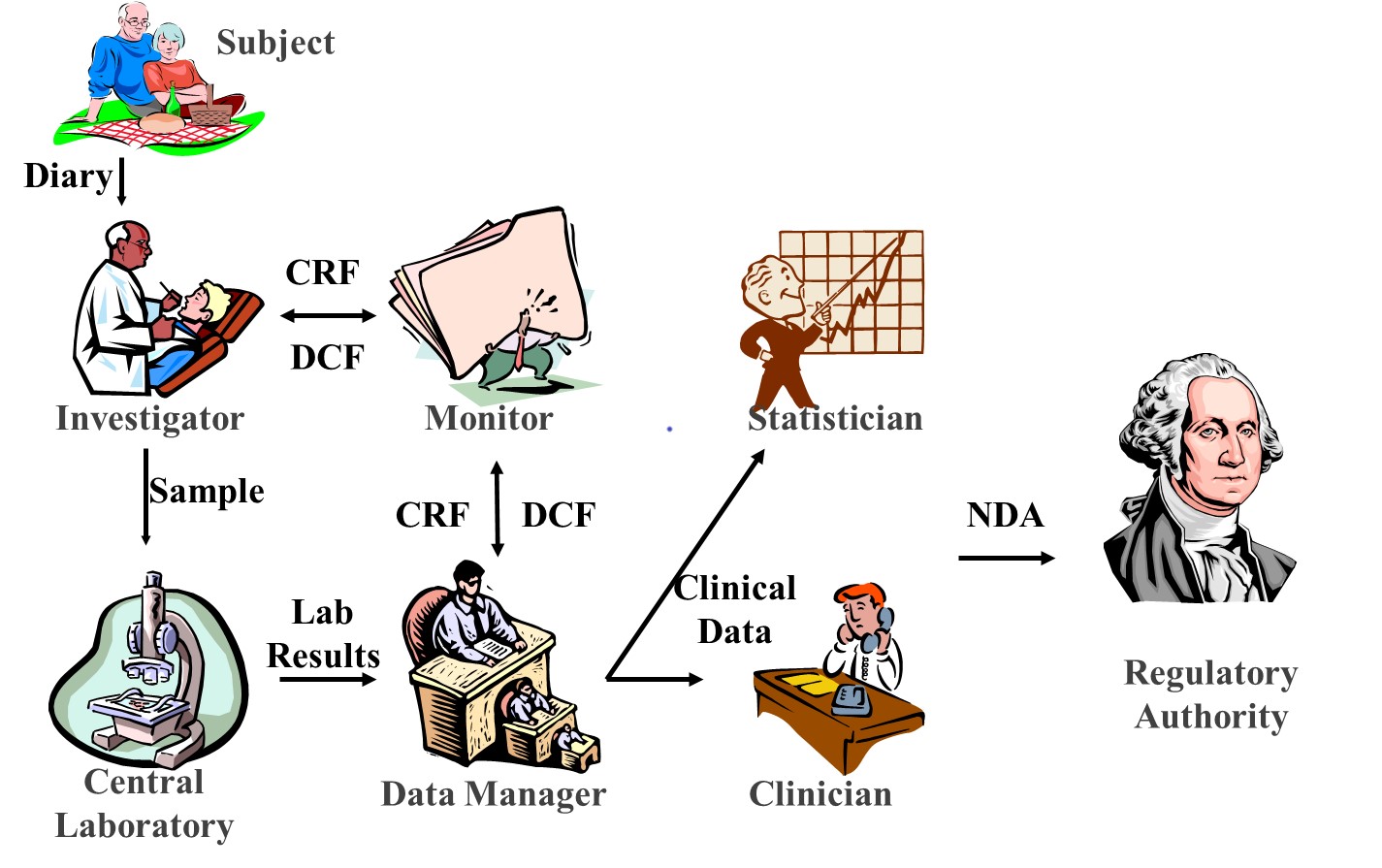
In the third issue of the "Clinical Study Pill" / “Pastila de Studii Clinice” section from Viata Medicala Magazine, we wrote about the clinical trials professionals who perform their entire activity during the research project around the patient to ensure that any newly invented medicine is safe for the patient and works for his benefit to improve the quality of life.
These research specialists are clinicians or laboratory doctors, imagists, physicists, biologists, biochemists, statisticians, representatives of regulatory agencies, specialists in writing research protocols (medical writers) etc. stick together for the same desire: improving the health of patients.
The article has issued on Viata Medicala no.5, from February 8, on page 148. See the article here.
The author is Dr Cristina Florescu Moraid, the founder and coordinator of AVANTYO Institute of Clinical Research.
***
The series of articles about clinical trials in Viata Medicala magazine in the section “Pastila de … studii clinice” / “The clinical trials … pill,” are part of our education and information activity in 2020. The articles are dedicated to physicians, especially those who have not yet come in contact with clinical trials.
Please check the News Section of our site to find the rest of the articles.
You might also find them posted on our social media pages, from Facebook and Linkedin.




